Viruses are both the villains and heroes of life as we know it
Ivan Erill, University of Maryland, Baltimore County
 |
| Bacteriophages are viruses that infect bacteria and play a potential role in the evolution of life. NANOCLUSTERING/SCIENCE PHOTO LIBRARY /Science Photo Library via Getty Images |
Many biologists like me believe there is, at least for one specific type of virus – namely, bacteriophages, or viruses that infect bacteria. When the DNA of these viruses is captured by a cell, it may contain instructions that enable that cell to perform new tricks.
The mighty power of bacterial viruses
Bacteriophages, or phages for short, keep bacterial populations in check, both on land and at sea. They kill up to 40% of the oceans’ bacteria every day, helping control bacterial blooms and redistribution of organic matter.
Their ability to selectively kill bacteria also has medical doctors excited. Natural and engineered phages have been successfully used to treat bacterial infections that do not respond to antibiotics. This process, known as phage therapy, could help fight antibiotic resistance.
Recent research points to another important function of phages: They may be nature’s ultimate genetic tinkerers, crafting novel genes that cells can retool to gain new functions.
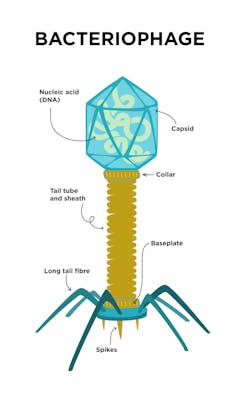 |
| Bacteriophage caspids can carry extra DNA that the virus can tinker with. Kristina Dukart/iStock via Getty Images Plus |
Phages are the most abundant life form on the planet, with a nonillion – that’s a 1 with 31 zeroes after it – of them floating around the world at any moment. Like all viruses, phages also have high replication and mutation rates, meaning they form many variants with different characteristics each time they reproduce.
Most phages have a rigid shell called a capsid that is filled with their genetic material. In many cases, the shell has more space than the phage needs to store the DNA essential for its replication. This means that phages have room to carry extra genetic baggage: genes that are not actually necessary for the phage’s survival that it can modify at will.
How bacteria retooled a viral switch
To see how this plays out, let’s take a deeper look at the phage life cycle.
Phages come in two main flavors: temperate and virulent. Virulent phages, like many other viruses, operate on an invade-replicate-kill program. They enter the cell, hijack its components, make copies of themselves and burst out.
Temperate phages, on the other hand, play the long game. They fuse their DNA with the cell’s and may lay dormant for years until something triggers their activation. Then they revert to virulent behavior: replicate and burst out.
Many temperate phages use DNA damage as their trigger. It’s sort of a “Houston, we have a problem” signal. If the cell’s DNA is being damaged, that means the DNA of the resident phage is likely to go next, so the phage wisely decides to jump ship. The genes that direct phages to replicate and burst out are turned off unless DNA damage is detected.
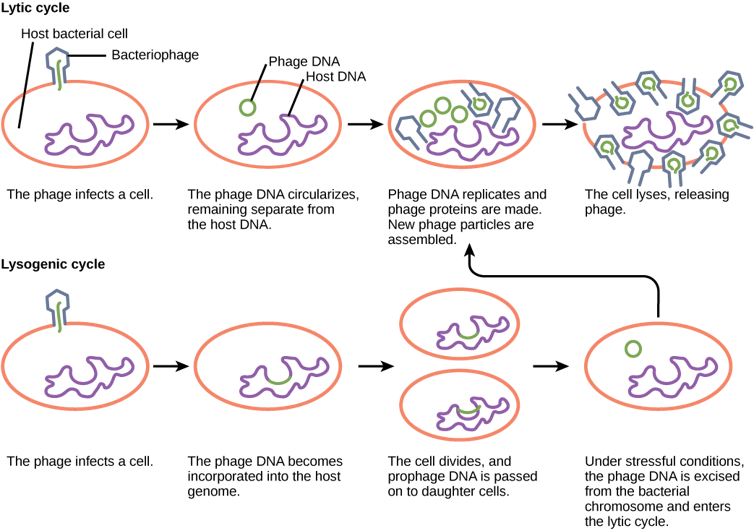
Bacteria have retooled the mechanisms controlling that life cycle to generate a complex genetic system that my collaborators and I have been studying for over two decades.
Bacterial cells are also interested in knowing if their DNA is getting busted. If it is, they activate a set of genes that attempt to repair the DNA. This is known as the bacterial SOS response because, if it fails, the cell is toast. Bacteria orchestrate the SOS response using a switch-like protein that responds to DNA damage: It turns on if there is damage and stays off if there isn’t.
Perhaps not surprisingly, bacterial and phage switches are evolutionarily related. This prompts the question: Who invented the switch, bacteria or viruses?
Our previous research and work by other researchers indicates that phages got there first. In our recent report, we discovered that the SOS response of Bacteroidetes, a group of bacteria that comprise up to a half of the bacteria living in your gut, is under control of a phage switch that was retooled to implement the bacteria’s own complex genetic programs. This suggests that bacterial SOS switches are in fact phage switches that got retooled eons ago.
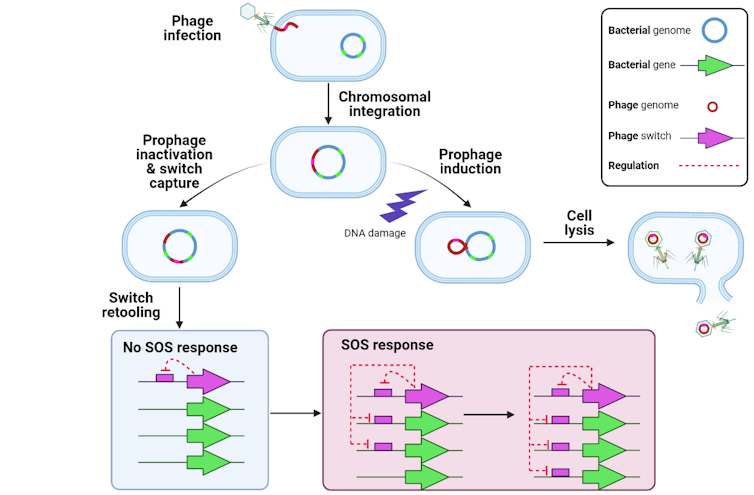
It’s not just bacterial switches that appear to be phage inventions. Beautiful detective work has shown that a bacterial gene needed for cell division also arose through “domestication” of a phage toxin gene. And many bacterial attack systems, such as toxins and the genetic guns used to inject them into cells, as well as the camouflage they use to evade the immune system, are known or suspected to have phage origins.
The upside of viruses
OK, you may think, phages are great, but the viruses that infect us are certainly not cool. Yet there is mounting evidence that the viruses that infect plants and animals are also a major source of genetic innovation in these organisms. Domesticated viral genes have been shown, for instance, to play a key role in the evolution of mammalian placentas and in keeping human skin moist.
Recent evidence suggests that even the nucleus of a cell, which houses DNA, could have also been a viral invention. Researchers have also speculated that the ancestors of today’s viruses may have pioneered the use of DNA as the primary molecule for life. Not a small feat.
So while you may be used to thinking of viruses as the quintessential villains, they are arguably nature’s powerhouses for genetic innovation. Humans are likely here today because of them.![]()
Ivan Erill, Associate Professor of Biological Sciences, University of Maryland, Baltimore County
This article is republished from The Conversation under a Creative Commons license. Read the original article.
[Research into coronavirus and other news from science Subscribe to The Conversation’s new science newsletter.]