Inform yourself
Benjamin Elling, Wesleyan University
 |
| Most plastic products that are clear and strong are made using bisphenol A, or BPA. Beton Studio/iStock via Getty Images |
At the time, the U.S. Food and Drug Administration concluded that some exposure was safe for adults. But other health agencies, including the European Food Safety Authority, have concluded that the levels of BPA the FDA considers safe may have adverse health effects for adults as well.
In early June 2022, the FDA signaled that it is reconsidering what amount of exposure to BPA is safe for adults, announcing that it would reconsider its guidance on the use of BPA in plastics that come into contact with food.
As a synthetic polymer chemist, I think a lot about how to design new polymers, with particular focus on how to do so sustainably. It’s natural to wonder why companies don’t simply replace BPA with another chemical if health is such a concern. The secret to what makes BPA such an irreplaceable ingredient in plastics is the same thing that leads to its health risks – the molecule’s chemical structure.
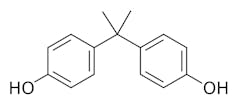 |
| Bisphenol A is made of two carbon rings with small alcohol groups attached and is used to produce strong, clear plastics. Darkness3560/Wikimedia Commons |
What is BPA?
BPA is a small molecule made of two carbon rings with a bonded oxygen and hydrogen attached to either end. BPA can react with other carbon-based molecules to form long chains, with the BPA molecules stitched together by small chemical links.
Nearly all of the BPA produced in the world is used to manufacture plastics, mostly a specific type called polycarbonate. BPA-derived polycarbonates are transparent, incredibly strong, light and don’t begin to melt or lose structural integrity until they reach very high temperatures. These properties make polycarbonates excellently suited for use in everything from the lenses of eyeglasses to water bottles.
It’s all about the structure
In chemistry, structure means everything. The reasons different materials have different properties is due to their chemical structure.
BPA polymers are rigid because the carbon rings in BPA molecules are themselves rigid. Compare this to polyethylene, the thin, flexible material used to make plastic bags. The long chains of repeating molecules that make up polyethylene are very flexible. So the plastics they produce are highly pliable, too.
 |
| BPA plastics are strong, transparent, light and have a high melting point, which makes them the perfect material for lenses for your eyeware. Nipitphon Na Chiangmai / EyeEm via Getty Images |
When BPA plastics are made, nearly all the individual molecules of BPA are chemically bound to the plastic. So most of the BPA that leaches out of food containers or water bottles results from the plastic slowly breaking down.
When BPA polycarbonates are exposed to water and heat – say, when you put a plastic bottle in your dishwasher – the chemical bonds that link these BPA molecules together can break down in a process known as hydrolysis. Because of its unique structure, BPA polycarbonates are generally more susceptible to hydrolysis than plastics like polyethylene.
Hydrolysis breaks down plastic at a chemical level, and this releases a small amount of BPA molecules into the environment. In one study, researchers found that the process of washing a polycarbonate bottle leached 0.2 to 0.3 milligrams of BPA into each liter of water. For context, this is hundreds of times less concentrated than the levels of calcium and sodium in drinking water.
The search for a BPA replacement
BPA is an endocrine disruptor, meaning it disrupts how hormones function in the body. Given the negative health effects of consuming BPA and the fact that it breaks down when exposed to water, chemists have been searching for replacements for years.
A major concern with designing new plastics is that swapping out BPA for another molecule may not get rid of the negative health effects. Just as the chemical structure of BPA determines the properties of the material, the structure is also what triggers the negative biological effects. Endocrine disruptors like BPA, due to their similar structures to natural hormones, can bind to and activate endocrine receptors.
Research has shown that structurally similar chemical replacements, such as bisphenol F, produce similar health effects as BPA.
It’s also not easy to swap in a new molecule that has a different chemical structure because the plastic will then lose the desirable characteristics of BPA polycarbonates. But there is some promising new research. One path of inquiry focuses on making polycarbonates by reacting rigid bio-based molecules with carbon dioxide gas.
Polycarbonates are a ubiquitious part of modern life. As researchers develop new materials, it is important to consider not only the health risks – as the EPA is doing with BPA – but the environmental effects as well.![]()
Benjamin Elling, Assistant Professor of Chemistry, Wesleyan University
This article is republished from The Conversation under a Creative Commons license. Read the original article.